The research focus of the Piston lab is the understanding of glucose-regulated hormone secretion from pancreatic islets. We use:
- State-of-the-art fluorescence imaging methods
- Quantitative microscopy
- Biochemical and molecular biological techniques
- Mathematical models
Over the last 20 years, we have developed instrumentation and probe technology for quantitative measurements broadly applicable to cell biology, tissue, physiology, and whole-organism imaging experiments. Many properties of intact islets match those seen in vivo, and we are focusing on methods that allow us to assay living islet functions quantitatively, and these studies are proving critical to advancing our understanding of the regulation of glucagon secretion from α-cells.

Current questions
What is the mechanism underlying brown adipose transplant restoration of euglycemia?
What are the mechanisms underlying the inhibition of glucagon secretion at high-glucose? How do gap junctions, paracrine inhibitors, and juxtacrine pathways regulate glucagon secretion?
How do GPCR ligands modulate islet cell metabolism, signaling, and hormone secretion?
How many signaling pathways can be simultaneously imaged in vivo using new microscopy techniques?
Studies of the molecular pathways of islet hormone secretion
The convergence of newly developed instrumentation and optical probes allows us to examine quantitatively dynamic processes within ever more complicated biological systems. These new tools are revolutionizing almost all fields of biomedical research, and we continue to optimize such methods and expand the scope of their applicability.
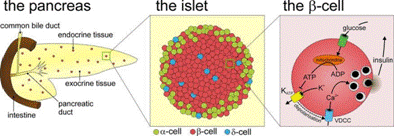
In our lab, research questions are focused on a model multicellular system, the islet of Langerhans, which is the functional unit responsible for glucose-stimulated insulin secretion and glucose inhibition of glucagon secretion. The islet contains glucagon secreting alpha-cells, insulin-secreting beta-cells, and somatostatin-secreting delta-cells, among others.
The long-term goal of our work is to understand at a quantitative molecular level the subcellular, cellular, and multicellular mechanisms of islet function, and its role in the regulation of blood glucose under normal and pathological conditions. Rotation projects are available in all the lab’s research areas.
The therapeutic success of insulin has led most islet research to focus on Beta-cells, although recent findings have strengthened our understanding of glucagon’s critical role in glucose homeostasis and the pathology of diabetes. Interactions with other islet cell types are thought to be involved in glucose-inhibition of glucagon secretion, since this inhibition is lost in vivo during Type 1 and advanced Type 2 diabetes, and in vitro when beta-cells are isolated from the islet. While many compounds have been shown to inhibit glucagon secretion from islets, no consensus model has been reached. The secretory product of delta-cells, somatostatin, is a promising molecular mediator of this interaction, but somatostatin alone does not inhibit glucagon secretion from isolated alpha-cells. This suggests that other cell-cell interactions within the islet are required to combine with somatostatin for proper function.
Using quantitative imaging methods and novel microfluidic devices, the dynamics of these molecular mechanisms can be followed in living cells within intact islets. These investigations utilize several available transgenic and tissue-specific knock-out mouse models with demonstrated phenotypes, as well as traditional biochemical and molecular biological approaches.